2009.05.29 -Germline and somatic JAK2 mutations and susceptibility to chronic myeloproliferative neoplasms
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2689447
Journal List > Genome Med > v.1(5); 2009
Genome Med. 2009; 1(5): 55.
PMCID: PMC268944
Published online 2009 May 29. doi: 10.1186/gm55.
Germline and somatic JAK2 mutations and susceptibility to chronic myeloproliferative neoplasms
Lynn R Goldin,1 Magnus Björkholm,2,4 Sigurdur Y Kristinsson,2 Jan Samuelsson,3,4 and Ola Landgren5
1Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892-7236, USA
2Department of Medicine, Division of Hematology, Karolinska University Hospital Solna and Karolinska Institutet, Stockholm, Sweden
3Department of Clinical Science and Education, Karolinska Institutet and Department of Internal Medicine, Stockholm South Hospital, Stockholm, Sweden
4Swedish Myeloproliferative Disorder Study Group, Stockholm, Sweden
5Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
Corresponding author.
Lynn R Goldin: goldinl@mail.nih.gov
Genome Med. 2009; 1(5): 55.
PMCID: PMC268944
Published online 2009 May 29. doi: 10.1186/gm55.
Abstract
Myeloproliferative neoplasms (MPNs) are a group of closely related stem-cell-derived clonal proliferative diseases. Most cases are sporadic but first-degree relatives of MPN patients have a five- to seven-fold increased risk for developing an MPN. The tumors of most patients carry a mutation in the Janus kinase 2 gene (JAK2V617F). Recently, three groups have described a strong association of JAK2 germline polymorphisms with MPN in patients positive for JAK2V617F. The somatic mutation occurs primarily on one particular germline JAK2 haplotype, which may account for as much as 50% of the risk to first-degree relatives. This finding provides new directions for unraveling the pathogenesis of MPN.
Cancer gene identification
Although many cancers show familial clustering, identification of the predisposing germline genes is challenging. Mutations in genes such as p53, BRCA1, CDKN2 and HNPCC causing high risk of tumors (often at an early age) were discovered from studies of rare families with striking tumor clusters [1]. However, these mutations explain only a small fraction of cancer susceptibility in the general population. More common cancers among older individuals also show familial aggregation and are probably the result of a combination of germline genes and environmental or other factors. The attempt to identify common susceptibility genes with smaller effects has been facilitated by the ability to conduct large-scale genome-wide association studies in cases and controls.
Somatic changes found in tumors may also be a result of germline susceptibility genes in the same region. Three recent articles in Nature Genetics [2–4] successfully demonstrate the potential of this approach by identifying a gene involved in susceptibility to developing chronic myeloproliferative neoplasms (MPNs) that is likely to have both germline and somatic effects. It is useful to review the recent history around this discovery and determine the lessons learned that could be applied to other cancers.
Genetics of myeloproliferative neoplasms
On the basis of the current World Health Organization criteria, MPNs include polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (MF) [5]. They are all stem-cell-derived clonal proliferative diseases whose shared and diverse phenotypic characteristics can be attributed to dysregulated signal transduction because of acquired somatic mutations [6]. They are uncommon tumors with yearly incident rates of 2.3 in 100,000 in the United States [7] and primarily affect older adults, with a variable clinical presentation. For example, approximately half of all MPN patients are reported to be asymptomatic at diagnosis [5,8]. Among PV and ET patients, the major symptoms are associated with hypertension or vascular abnormalities, including an increased risk for thromboembolismand hemorrhage [9,10]. Also, patients with PV and ET have an excess risk of developing MF, or transformation to myelodysplastic syndrome or leukemia [9,10]. Although available information is limited, life expectancy of patients with ET has been reported to be similar to that of the general population; however, for PV patients, life expectancy has been observed to be reduced compared with the general population [11]. In contrast, primary MF patients have an average survival of less than 5 years [12]. Currently, the underlying causes of MPNs are mostly unknown.
A mutation in the gene for Janus kinase 2 (JAK2V617F) is present in erythropoietin-independent erythroid colonies in PV and gives a proliferative advantage in these cells. The mutation is present in 95% of PV patients and in approximately 50% of ET and MF patients [6]. However, given that case studies have shown that only a minority of granulocytes affected by a chromosome 20q deletion also harbored JAK2V617F, other primary pathogenetic factors have been suggested. It has also been demonstrated that rare somatic events, such as a chromosome 20q deletion or loss of heterozygosity on 9p, have occurred more than once in subclones from the same patients, suggesting that the myeloproliferative disorder clone carries a predisposition to acquiring such genetic alteration [13]. A role for genetic factors in the etiology of MPNs has alsobeen suggested from case reports and smaller case series showing evidence of familial clustering of PV, ET, MF and chronic myeloid leukemia (CML) [14,15]. One study of patients from 72 families with different MPNs found no evidence for the JAK2V617Fmutation in the germline of patients who carried the mutation in their tumor cells, and the authors concluded that the JAK2 gene was a later ‘hit’ in these families [16].
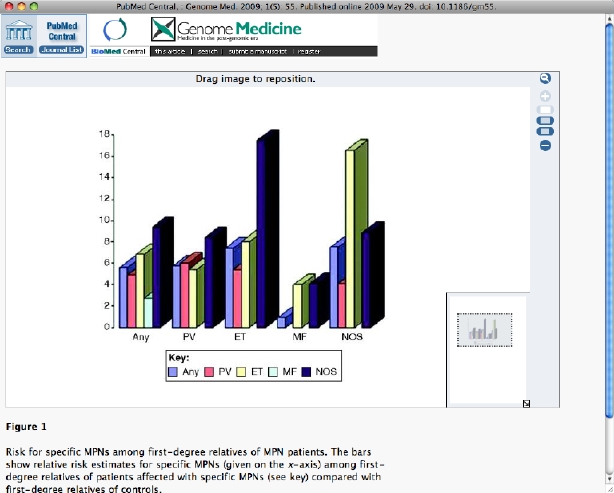
We recently published a study [17] using Swedish Registry data, showing that the risk of MPN in nearly 25,000 first-degree relatives of 11,000 cases was significantly higher (five- to seven-fold elevated) than in first-degree relatives of control individuals. The results are illustrated in Figure 1. We also found an approximately two-fold relative risk (borderline significant) for CML in relatives of MPN patients. In fact, a series of case reports has described co-existence of the JAK2 mutation and the BCR-ABL translocation, a chromosomal abnormality associated with CML [18]. This suggests that there is a strong important hereditary component to MPN above and beyond the rare ‘heavily loaded’ families. We found elevated risks for PV, ET and MF among relatives of MPN patients, suggesting that there is common genetic susceptibility among the subtypes. We emphasized in that article [17] that the high recurrence risks in relatives made the search for germline risk genes compelli
###
Figure 1
Risk for specific MPNs among first-degree relatives of MPN patients. The bars show relative risk estimates for specific MPNs (given on the x-axis) among first-degree relatives of patients affected with specific MPNs (see key) compared with first-degree relatives of controls.
Genome Med. 2009; 1(5): 55.
Published online 2009 May 29. doi: 10.1186/gm55.
###
We did not have to wait long for the identification of a germline susceptibility gene. The results from the three recent studies all converge on the conclusion that the JAK2 gene is a susceptibility gene for MPNs both in the germline and somatically [2–4]. These studies demonstrated that the malignant clone containing the JAK2 mutation was strongly associated with a particular haplotype defined by nearby single nucleotide polymorphisms (SNPs) in patients that were JAK2V617F-positive. The study by Jones et al. [2] demonstrated that a single haplotype was present in clones that were homozygous for JAK2V617F. The association was also found in a larger population of patients. Interestingly, in one family with a grandparent and grandchild both with PV, only one of the cases had the at-risk haplotype. One of the associated SNPs was also shown to be in cis with the JAK2V617F allele in the study by Olcaydu et al. [4]. Another nearby SNP was almost always found in cis with JAK2V617F in the study by Kilpivaara et al. [3] In all three studies, these SNPs showed high relative risks when JAK2V617F-positive patients were compared with controls. Kilpivaara et al. [3] also presented association of genome-wide markers in cases and controls and showed that the JAK2 SNP was highly significant even in the context of a genome-wide search. The article by Jones et al. [2] showed weak evidence that the risk haplotype was also associated with JAK2V617F-negative patients.
Future directions
The evidence is now in favor of the JAK2 locus being an important germline and somatic hit in the pathology of MPNs. The authors of the three studies [2–4] have speculated possible mechanisms. One possibility is that the associated SNPs are in linkage disequilibrium with an unidentified functional variant. Other possibilities are that the associated haplotype causes the JAK2 gene to be more susceptible to mutation or allows increased survival of JAK2V617F alleles.
But what about the high-risk families that have been described? As mentioned above, Bellanné-Chantelot et al. [16] did not find the JAK2V617Fmutation in the germline in MPN families and also did not see evidence for linkage to the JAK2 region of chromosome 9. It is possible that the JAK2 region has a role in some families but that other rare loci also exist. It is also likely that there are other common genes that contribute to risk in the population. Jones et al. [2] estimate that the JAK2-associated haplotype accounts for 50% of the risk in first-degree relatives on the basis of our relative risk estimate of 5.7 in first-degree relatives of PV patients [17]. Thus, there are likely to be additional common and rare susceptibility genes. Nevertheless, these new findings are a large step in the progress towards understanding the pathogenesis of MPN.
Abbreviations
CML: chronic myeloid leukemia; ET: essential thrombocythemia; MF: myelofibrosis; MPN: myeloproliferative neoplasm; PV: polycythemia vera; SNP: single nucleotide polymorphism.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LRG and OL wrote the report. All authors read, gave comments and approved the final version of the manuscript.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health National Cancer Institute and by grants from the Swedish Cancer Society, Stockholm County Council, the Karolinska Institutet Foundations.
References
- Darbary H, Stoler DL, Anderson GR. Family cancer syndromes: inherited deficiencies in systems for the maintenance of genomic integrity. Surg Oncol Clin N Am. 2009;18:1–17. doi: 10.1016/j.soc.2008.08.001. vii. [PubMed]
- Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, Cario H, Pahl HL, Collins A, Reiter A, Grand F, Cross NC. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41:446–449. doi: 10.1038/ng.334. [PubMed]
- Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, Bass A, Marubayashi S, Heguy A, Garcia-Manero G, Kantarjian H, Offit K, Stone RM, Gilliland DG, Klein RJ, Levine RL. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41:455–459. doi: 10.1038/ng.342. [PubMed]
- Olcaydu D, Harutyunyan A, Jager R, Berg T, Gisslinger B, Pabinger I, Gisslinger H, Kralovics R. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41:450–454. doi: 10.1038/ng.341. [PubMed]
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. doi: 10.1038/sj.leu.2404955. [PubMed]
- Tefferi A, Gilliland DG. Oncogenes in myeloproliferative disorders. Cell Cycle. 2007;6:550–566. [PubMed]
- Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK., (Eds). SEER Cancer Statistics Review, 1975-2005. Bethesda: National Cancer Institute; 2008.
- Sanchez S, Ewton A. Essential thrombocythemia: a review of diagnostic and pathologic features. Arch Pathol Lab Med. 2006;130:1144–1150. [PubMed]
- Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost. 2007;33:313–320. doi: 10.1055/s-2007-976165. [PubMed]
- Tefferi A, Gangat N, Wolanskyj AP, Schwager S, Pardanani A, Lasho TL, Mesa R, McClure RF, Li CY, Hanson CA. 20+ years without leukemic or fibrotic transformation in essential thrombocythemia or polycythemia vera: predictors at diagnosis. Eur J Haematol. 2008;80:386–390. doi: 10.1111/j.1600-0609.2008.01038.x. [PubMed]
- Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, Orlandi E, Arcaini L, Brusamolino E, Pascutto C, Cazzola M, Morra E, Lazzarino M. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [PubMed]
- Hoffman R, Rondelli D. Biology and treatment of primary myelofibrosis. Hematology Am Soc Hematol Educ Program. 2007;2007:346–354. [PubMed]
- Schaub FX, Jager R, Looser R, Hao-Shen H, Hermouet S, Girodon F, Tichelli A, Gisslinger H, Kralovics R, Skoda RC. Clonal analysis of deletions on chromosome 20q and JAK2-V617F in MPD suggests that del20q acts independently and is not one of the predisposing mutations for JAK2-V617F. Blood. 2009;113:2022–2027. doi: 10.1182/blood-2008-07-167056. [PubMed]
- Rumi E. Familial chronic myeloproliferative disorders: the state of the art. Hematol Oncol. 2008;26:131–138. doi: 10.1002/hon.863. [PubMed]
- Rumi E, Passamonti F, Picone C, Della Porta MG, Pascutto C, Cazzola M, Lazzarino M. Disease anticipation in familial myeloproliferative neoplasms. Blood. 2008;112:2587–2588. doi: 10.1182/blood-2008-05-160739. author reply 2588-2589. [PubMed]
- Bellanné-Chantelot C, Chaumarel I, Labopin M, Bellanger F, Barbu V, De Toma C, Delhommeau F, Casadevall N, Vainchenker W, Thomas G, Najman A. Genetic and clinical implications of the Val617Phe JAK2 mutation in 72 families with myeloproliferative disorders. Blood. 2006;108:346–352. doi: 10.1182/blood-2005-12-4852. [PubMed]
- Landgren O, Goldin LR, Kristinsson SY, Helgadottir EA, Samuelsson J, Bjorkholm M. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112:2199–2204. doi: 10.1182/blood-2008-03-143602. [PubMed]
- Kramer A. JAK2-V617F and BCR-ABL-double jeopardy? Leuk Res. 2008;32:1489–1490. doi: 10.1016/j.leukres.2008.03.011. [PubMed]